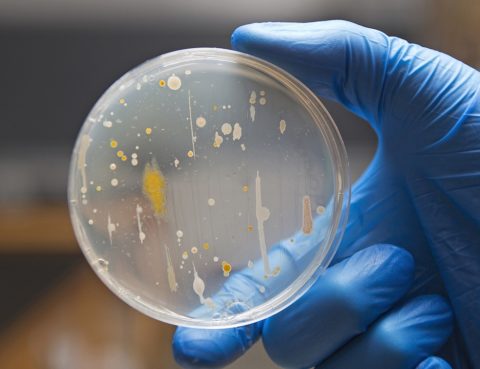
The presence of microorganisms in the cosmetic may cause its delamination, change of fragrance, and loss of its properties. Furthermore, a contaminated cosmetic product poses a threat to consumer health. Therefore the quality of cosmetics available on the EU market is regulated by law. According to the Regulation (EC) No. 1223/2009 of the European Parliament…
Examination of microbiological purity is next to the stress tests, and the stability test, one of the conditions for the admission of a cosmetic product for sale in the European Union countries. The effects of microorganisms on the cosmetic. Contamination of a cosmetic product with microorganisms has a number of consequences. As a result of…
Microbiological purity tests of cosmetics are an important element for the development of the most important document proving the quality of a given product, which is the cosmetic product safety report. On its basis one can conclude on the safety of a given cosmetic product placed on the market by the producer. The requirement to…
Registration of cosmetics on the European Union market is regulated by the Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products, applicable as from July 11, 2013. The Ordinance replaced the provisions of the Cosmetics Act (Journal of Laws 2001 No. 72, item 473, as…
Regulation (EC) No. 1223/2009 of the European Parliament has resulted in a unification of the registration of cosmetics in the countries of the European Union. From 11 January 2012, persons responsible for cosmetics have the possibility to complete the information referred to in Article 13 of Regulation (EC) No. 1223/2009 to the online Cosmetic Products…