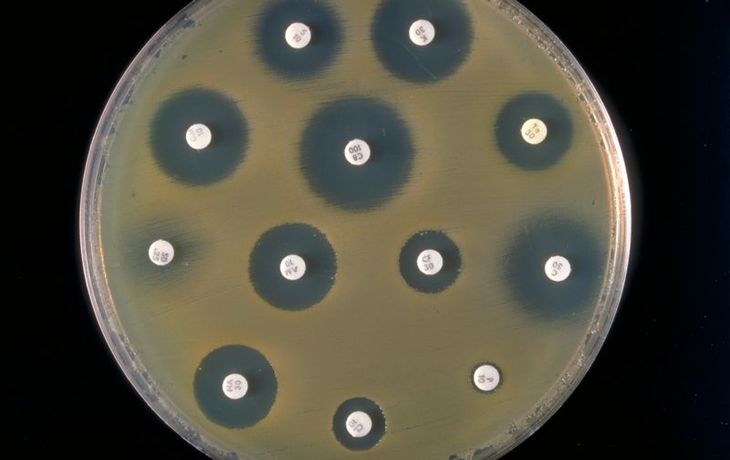
Bacteria belonging to the order Enterobacterales constitute a large, closely related group of Gram-negative bacilli. They include among others: Escherichia coli, Klebsiella pneumoniae and Salmonella spp. Many bacteria belonging to this order constitute the bacterial flora of the intestines of healthy person. Some species are absolute pathogens. Due to their close relationship, these microorganisms share a number of similarities. One of these is the ability to produce β-lactamases, enzymes that destabilise the β-lactam bond in the β-lactam antibiotic molecule. These antibiotics include penicillins, cephalosporins, carbapenems and monobactams.
History of antibiotics
The discovery of the bactericidal properties of penicillin by Alexander Fleming in 1928 marked the beginning of a new era in medicine. This first antibiotic was introduced to pharmacies in 1946. Even before its introduction to the market, reports of Escherichia coli strains capable of producing penicillinase inactivating this antibiotic appeared. The phenomenon of the spread of penicillin resistance mechanisms was also documented very early on in Staphylococcus aureus and subsequently in other bacterial species. Initially, antibiotic-resistant strains were detected only amongst patients in hospitals. Nevertheless, antibiotic-resistant bacteria soon appeared outside hospitals as well. Furthermore, the years 1940-1960 are called the golden age of antibiotics. During this period, new antibiotics were introduced and a wide range of them allowed resistance mechanisms to be circumvented and effective therapy to be provided. The introduction of new generations of penicillins as well as other antibiotics went hand in hand with the development of resistance among bacterial cells. Since the discovery of penicillin, more than 150 antibiotics have been found, and resistance mechanisms have emerged for most of them.
Causes of bacterial antibiotic resistance
Bacterial resistance to antibiotics allows bacteria, yeasts and moulds to survive in their environment. These substances are produced by them as defence mechanisms and allow them to compete for space and food resources with other microorganisms in the environment. The mechanisms determining bacterial resistance to antibiotics arise mainly as a result of spontaneous mutations and the acquisition of genetic information as a result of horizontal gene transfer (HGT). The genes responsible for resistance are privileged in the process of natural selection. The extensive use of antibiotics in both medicine, agriculture and animal husbandry results in the selection of resistant strains. This includes the use of prescription drugs to combat diseases that do not require this group of drugs. An example is the administration of antibiotics for colds or flu, which are viral infections. In recent years, attention has been drawn to the particular significance of the overuse of antibiotics in agriculture and animal husbandry. It is estimated that approximately 80% of antibiotics in the US are marketed for infection control, growth promotion, and weight gain in livestock. These substances are called antibiotic growth promoters (AGP). However, these substances belong to the same groups of chemical compounds as the antibiotics used in medicine. That is why such a wide use of AGP has significantly contributed to the development of antibiotic resistance of bacteria, including those pathogenic for humans. The spread of resistant strains is greatly influenced by contamination of the environment with antibiotic growth stimulants, which enter the ecosystem along with the excrements of farm animals.
Extended-spectrum β -lactamases (ESBLs)
One of the most significant mechanisms of antibiotic resistance in Enterobacterales is the production of hydrolysable enzymes: penicillins, cephalosporins and monobactams. These enzymes are called extended spectrum β-actamases (ESBLs). The mechanism was discovered in 1983 in Klebsiella pneumoniae. Initially, ESBL bacteria were known only as etiological agents of hospital infections. Nowadays, they are also detected as the substrate of extra-hospital infections. What is more, carriers of these bacteria are also known.
New Delhi metallo-β-lactamase (NDM-1)
The mechanism was first diagnosed in 2008 in Klebsiella pneumoniae and Escherichia coli. Its name comes from the city where the patient was located, from which the first NDM-1 positive bacteria were isolated. The described mechanism is based on the production of an enzyme from the group of metallo-β-lactamases. It makes bacteria resistant to many beta-lactam antibiotics, including carbapenems. Carbapenem antibiotics are considered drugs of last resort. Mainly used to treat diseases caused by bacteria that are resistant to other antibiotics. The substances of this group are of particular importance also due to their broad spectrum of activity. Carbapenems are active against both gram-positive and gram-negative aerobic and anaerobic pathogenic bacteria.
Undiagnosed resistance of pathogens to a number of antibiotics is often the cause of long and ineffective treatment of infections. They are often very dangerous and can lead to complications and even death of an infected person.
